
Pulpdent est une entreprise familiale de recherche et de fabrication de produits dentaires créée en 1947 et engagée dans ses principes fondateurs d’éducation, de prévention et de soins dentaires proactifs afin que les gens puissent vivre une vie plus saine et plus productive.
La recherche et le développement de produits de Pulpdent® visent à libérer les pouvoirs de guérison de la nature avec des matériaux bioactifs qui imitent les propriétés physiques et chimiques de la structure dentaire, se comportent favorablement dans l’environnement buccal humide, et maximisent le potentiel de reminéralisation.


Principes fondateurs
Le fondateur de Pulpdent, le Dr Harold Berk, a exercé la dentisterie pendant près de 65 ans et a enseigné à la faculté de médecine dentaire de l’université de Tufts de 1946 à 2005. Les principes fondamentaux du Dr Berk continuent de guider l’entreprise :
Les produits Pulpdent® sont fabriqués à Watertown, dans le Massachusetts, aux États-Unis, conformément au système de qualité de l’entreprise et à toutes les exigences réglementaires. Chaque année, nous renouvelons notre engagement à investir dans la recherche et les nouvelles technologies, à gagner la confiance de la profession, à inspirer les cliniciens avec de nouvelles idées et de nouveaux matériaux, et à fournir le leadership nécessaire pour atteindre ces objectifs.
Distribution mondiale
PulpdentLe portefeuille de produits en expansion de la société est distribué dans le monde entier par l’intermédiaire de partenaires de distribution locaux. Sa croissance lui a permis d’étendre la portée de ses produits uniques qui font progresser la norme de soins.
Prix et reconnaissance
Près d’un siècle d’histoire
PulpdentL’histoire de la société est ancrée dans un engagement en faveur de la recherche dentaire originale. Au fil des ans, cette recherche a débouché sur des technologies chimiques brevetées et des matériaux dentaires SMART™ qui améliorent activement la santé bucco-dentaire et modifient le paradigme de l’industrie en ce qui concerne les normes relatives aux matériaux dentaires.
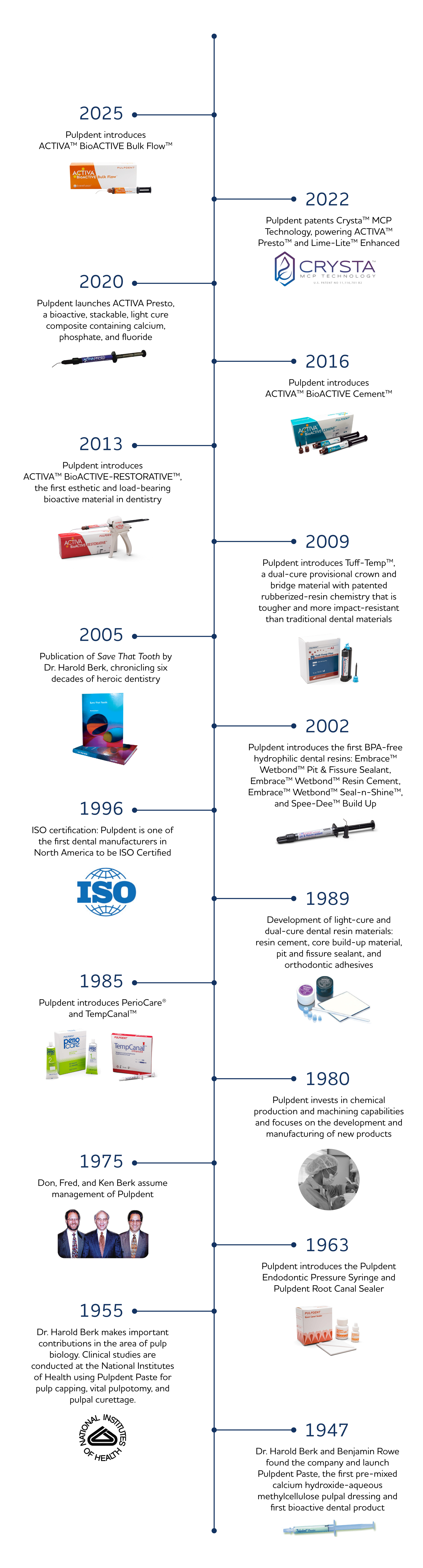
Ligne de temps de Pulpdent®.
Survolez chaque date pour en savoir plus
1947
Dr. Harold Berk and Benjamin Rowe found the company and launch Pulpdent Paste, the first pre-mixed calcium hydroxide-aqueous methylcellulose pulpal dressing and first bioactive dental product

1955
Dr. Harold Berk makes important contributions in the area of pulp biology. Clinical studies are conducted at the National Institutes of Health using Pulpdent Paste for pulp capping, vital pulpotomy, and pulpal curettage.

1963
Pulpdent introduces the Pulpdent Endodontic Pressure Syringe and Pulpdent Root Canal Sealer

1975
Don, Fred, and Ken Berk assume management of Pulpdent

1980
Pulpdent invests in chemical production and machining capabilities and focuses on the development and manufacturing of new products

1985
Pulpdent introduces PerioCare® and TempCanal™


1989
Development of light-cure and dual-cure dental resin materials: resin cement, core build-up material, pit and fissure sealant, and orthodontic adhesives

1996
ISO certification: Pulpdent is one of the first dental manufacturers in North America to be ISO Certified

2002
Pulpdent introduces the first BPA-free hydrophilic dental resins: Embrace™ Wetbond™ Pit & Fissure Sealant, Embrace™ Wetbond™ Resin Cement, Embrace™ Wetbond™ Seal-n-Shine™, and Spee-Dee™ Build Up

2005
Publication of Save That Tooth by Dr. Harold Berk, chronicling six decades of heroic dentistry

2009
Pulpdent introduces Tuff-Temp™, a dual-cure provisional crown and bridge material with patented rubberized-resin chemistry that is tougher and more impact-resistant than traditional dental materials

2013
Pulpdent introduces ACTIVA™ BioACTIVE-RESTORATIVE™, the first esthetic and load-bearing bioactive material in dentistry

2016
Pulpdent introduces ACTIVA™ BioACTIVE Cement™

2020
Pulpdent launches ACTIVA Presto, a bioactive, stackable, light cure composite containing calcium, phosphate, and fluoride

2022
Pulpdent introduces Pulpdent patents Crysta™ MCP Technology, powering ACTIVA™ Presto™ and Lime-Lite™ Enhanced

2025
Pulpdent introduces ACTIVA™ BioACTIVE Bulk Flow™
































Responsabilité sociale
Pulpdent® a toujours porté un intérêt particulier à la santé publique et à la santé bucco-dentaire des enfants. Nous coopérons avec la communauté de la santé publique et soutenons de nombreuses initiatives de santé publique par le biais du programme de partenariat de santé publique de Pulpdent®. Pour demander un don en nature pour une initiative de santé publique , cliquez ici.